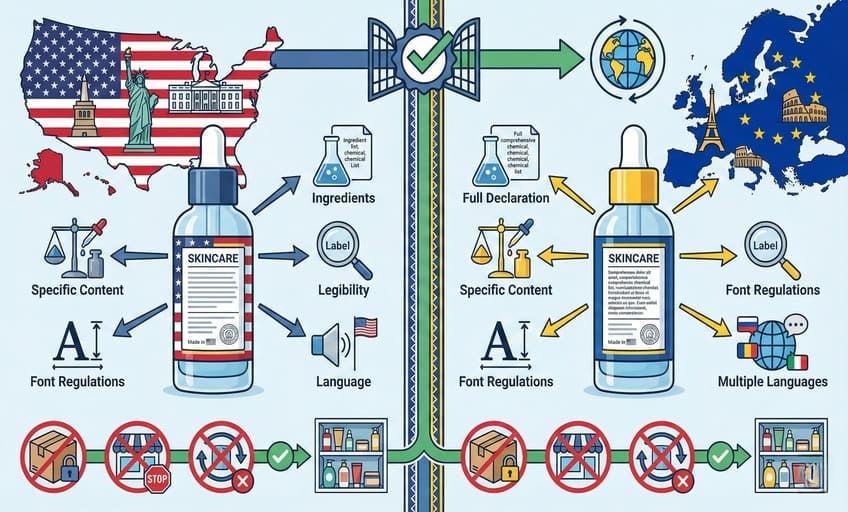
Failing to meet label rules1 can halt your launch, block sales, or cause recalls—compliance is the first step before any skincare hits the shelf.
US and EU regulations2 demand clear, accurate labeling with complete ingredient lists3, specific content, and legible details4—including strict font, language, and packaging guidelines5 for each market.
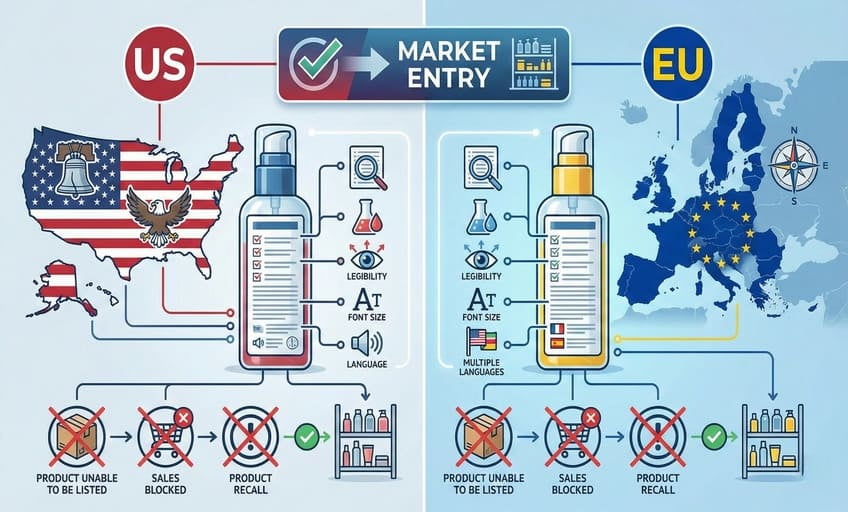
At ECO-BAMBOO, I help clients get this right before anything goes to print. I’ve seen brands spend months on packaging, only to scramble for a re-run after missing a small rule. Labels must tell the full truth: what’s inside, how much, who made it, and how to use it safely. This information protects not just your business, but your customer. That’s why thorough compliance is as crucial as design in making sustainable, beautiful packaging succeed in any global market.
What are the FDA requirements6 for product labeling?
The FDA requires all skincare labels to show product identity, net quantity, distributor/manufacturer details, and a full, INCI-standard ingredient list—in easily readable fonts and with honest, non-misleading statements.
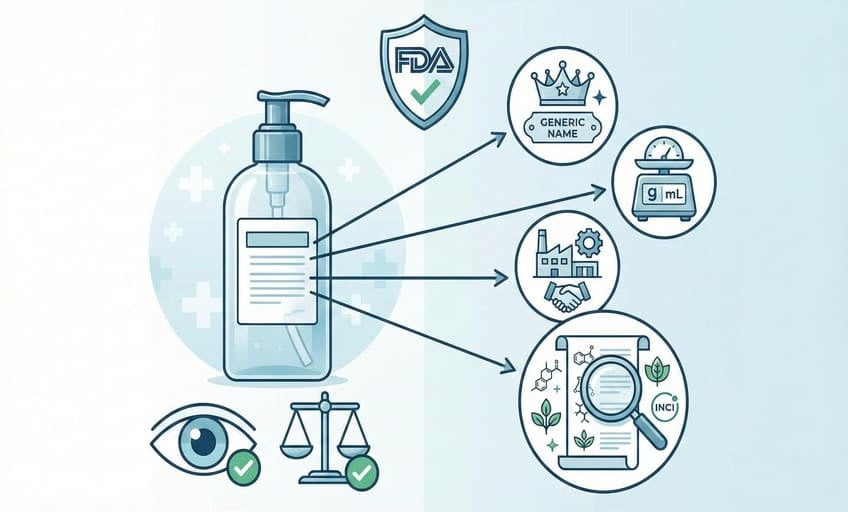
I always check FDA guides line by line for every new US-bound project. The main points cover what the product is, who made or distributed it, how much is inside, and every ingredient—listed from most to least, using INCI names7. It’s essential to use a font size that’s easy to read, usually at least 1/16 inch for most containers. Any claim (“all natural”, “made in USA”) must be true and scientifically supportable. Avoiding tricky wording is the only way to avoid recourse or delays. Getting it right builds trust and speeds up market entry.
FDA Labeling Checklist
| Requirement | Description |
|---|---|
| Identity of product | What the item is (“Moisturizer Cream”) |
| Net contents | Amount in weight/volume (“50ml”, “2 fl. oz.”) |
| Manufacturer info | Name and address—can be direct manufacturer or distributor |
| Ingredient list | All materials, descending order, INCI names7 |
| Claims | Must be honest, non-misleading, and defensible |
| Legibility | Font size minimum, clear contrast |
Do you have to list all ingredients in skin care?
Yes, US and EU law require all skincare ingredients to be listed on the label in descending order of weight, using internationally recognized names—no hidden or omitted substances allowed.
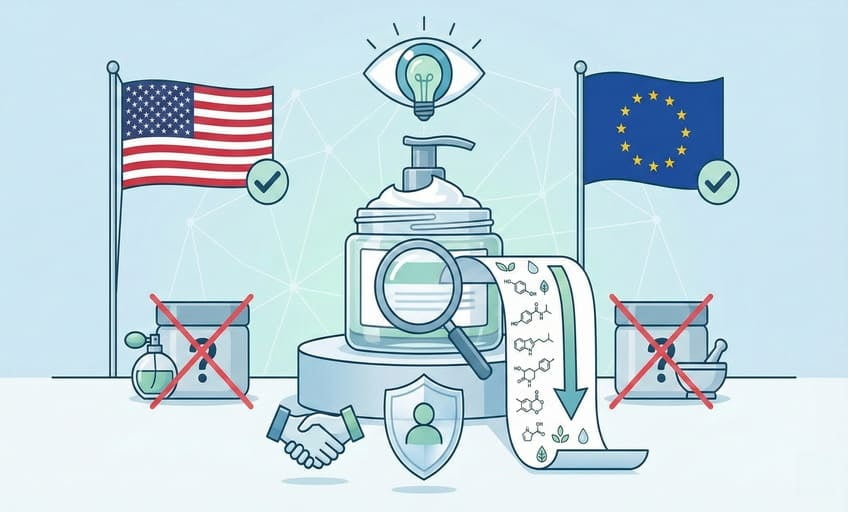
Clients often ask if “fragrance” or “base” can cover multiple materials. The answer is simple: transparency8 wins. Regulations don’t just demand listing all ingredients—they insist on using their official INCI (International Nomenclature of Cosmetic Ingredients) names. There are very few exceptions, such as trade-secret fragrance blends (still under strict limits). For the US and EU, if it’s in there, it goes on the label. This rule guarantees consumer safety and brand accountability.
Ingredient List Table
| Region | Ingredient Listing Requirement | Special Notes |
|---|---|---|
| US | All, by weight, using INCI names7 | Fragrance can be grouped |
| EU | All, by weight, using INCI names7 | Allergens must also be shown |
What must appear on a skincare product label in the EU?
EU labels must display product identity, net content, ingredient list, country of origin, batch number9, PAO, responsible party info10, usage directions, and caution statements—all in the relevant market language.
When guiding B2B partners through EU launches, I point straight to Regulation (EC) No 1223/2009. Every package must show what the product is, where it comes from, and who takes responsibility. The “period after opening11” (like “12M” for twelve months) tells buyers how long the product stays safe. Ingredients must be fully listed, with allergens highlighted, and users need clear directions—often in several languages for pan-European sales. Tight rules also govern batch codes, for tracking and recall. These details help buyers feel informed and protected.
EU Label Content Table
| Must Have on Label | Description/Example |
|---|---|
| Identity of product | (“Facial serum”) |
| Net quantity | (“30ml”) |
| Ingredient list | Full, in INCI order |
| Country of origin | (“Made in France”) |
| Batch code | (“B012345”) |
| Responsible person | Company details in EU |
| PAO (“Period After Opening”) | (Example: “12M” icon) |
| Directions/warnings | (“For external use only”) |
| Languages | Local official language(s) |
What are the language and font requirements12 for cosmetic labels13 in the EU?
EU cosmetic labels13 must use clear, indelible lettering with required info in the official language(s) of each target country—minimum font size and high contrast are strictly enforced.
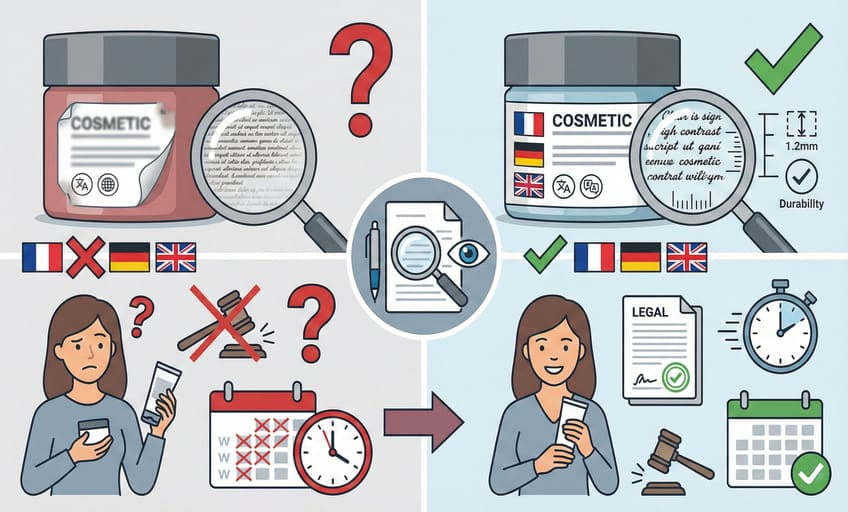
With printed bamboo jars, ECO-BAMBOO solves the challenge by using high-contrast, laser-engraved or hot-stamped text that passes both SGS and EU visibility standards. Labels must be permanent so they don’t rub off, and readable at a glance—usually minimum height of 1.2mm for essential data. If you sell in France and Germany, everything must appear in French and German, not just English. This extra work reassures end users and cuts legal risk. The final step? Always proof every detail before final production—one missed word or hard-to-read font can cost weeks.
EU Label Font and Language Table
| Requirement | Detail |
|---|---|
| Font size | At least 1.2 mm height for core info |
| Font style | Clear, contrasting, permanent ink/imprint |
| Language(s) | Official language(s) of destination country |
| Label durability | Text must be indelible and tamper-resistant |
Conclusion
US and EU skincare packaging must follow strict, detailed label rules1—clear content, full ingredients, readable text, and local language—ensuring safe, market-ready products for global customers.
Understanding label rules is crucial for compliance and successful product launches in the skincare industry. ↩
Explore these regulations to ensure your skincare products meet legal requirements and avoid costly recalls. ↩
Learn about the importance of complete ingredient lists for transparency and consumer safety. ↩
Understanding legibility requirements helps in creating labels that are clear and compliant. ↩
Explore packaging guidelines to ensure your products are compliant and appealing to consumers. ↩
Familiarize yourself with FDA requirements to ensure your products are compliant and market-ready. ↩
Discover how INCI names enhance clarity and compliance in ingredient labeling. ↩
Explore how transparency builds trust with consumers and enhances brand accountability. ↩
Learn how batch numbers aid in product tracking and safety recalls. ↩
Discover the importance of including responsible party information for consumer trust and legal compliance. ↩
Understanding PAO helps consumers know how long products remain safe to use. ↩
Learn about font requirements to ensure your labels are readable and compliant. ↩
Understanding key elements of cosmetic labels is essential for compliance and effective marketing. ↩